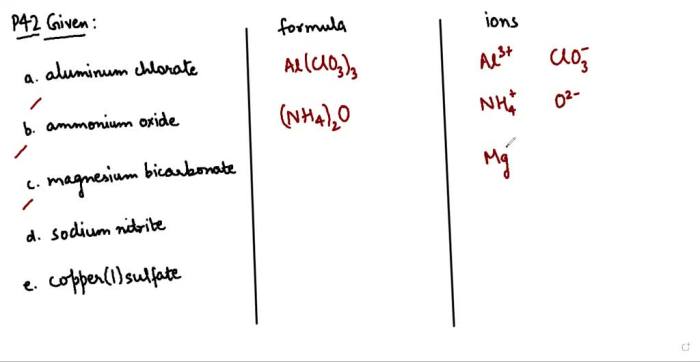
Balancing chemical equations worksheet 2 – Embark on a journey to master the art of balancing chemical equations with our comprehensive guide and Worksheet 2. Delve into the intricacies of this fundamental chemistry concept, unravel its importance, and gain a deep understanding through practical examples and step-by-step solutions.
Introduction
Balancing chemical equations is a fundamental skill in chemistry. It involves adjusting the coefficients in a chemical equation to ensure that the number of atoms of each element is the same on both sides of the equation.
Balancing chemical equations is important because it allows us to:
- Predict the products of a chemical reaction
- Calculate the stoichiometry of a reaction (the mole ratios of the reactants and products)
- Understand the mechanisms of chemical reactions
An unbalanced chemical equation is one in which the number of atoms of each element is not the same on both sides of the equation. A balanced chemical equation is one in which the number of atoms of each element is the same on both sides of the equation.
Balancing Chemical Equations: Balancing Chemical Equations Worksheet 2
There are several methods that can be used to balance chemical equations. The most common method is the oxidation-reduction method, which is based on the principle that the total number of electrons lost must be equal to the total number of electrons gained.
To balance a chemical equation using the oxidation-reduction method, follow these steps:
- Identify the oxidation and reduction half-reactions.
- Balance the half-reactions in terms of mass and charge.
- Multiply the half-reactions by appropriate factors so that the number of electrons lost is equal to the number of electrons gained.
- Add the balanced half-reactions together to get the overall balanced equation.
Here is an example of how to balance a chemical equation using the oxidation-reduction method:
Fe + HCl → FeCl2+ H 2
The oxidation half-reaction is:
Fe → Fe2++ 2 e –
The reduction half-reaction is:
H++ 2 e –→ H 2
Multiplying the oxidation half-reaction by 2 and the reduction half-reaction by 1 gives:
Fe → 2 Fe2++ 4 e –
H++ 2 e –→ H 2
Adding the two half-reactions together gives the overall balanced equation:
Fe + 2 HCl → 2 FeCl2+ H 2
FAQ Overview
What is the purpose of balancing chemical equations?
Balancing chemical equations ensures that the number of atoms of each element on the reactants’ side equals the number of atoms of that element on the products’ side, reflecting the law of conservation of mass.
How do I approach balancing chemical equations?
Follow a systematic approach: identify unbalanced equations, adjust coefficients in front of reactants and products, check for balance, and repeat until all elements are balanced.
What common mistakes should I avoid when balancing equations?
Avoid changing subscripts (elemental formulas), balancing equations by changing the chemical formulas, or introducing new substances not present in the original equation.