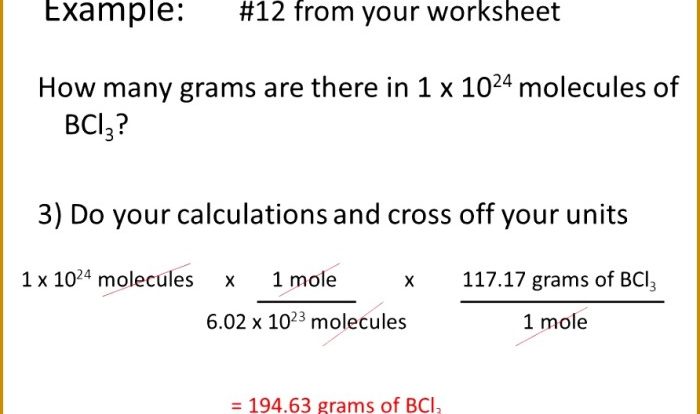
Aluminum chlorate + barium sulfate aluminum sulfate + barium chlorate – Aluminum chlorate and barium sulfate, when combined, yield aluminum sulfate and barium chlorate, initiating a captivating chemical journey. This in-depth exploration delves into the intricacies of these substances, their properties, reactions, and practical applications, unraveling a world of scientific intrigue.
From the molecular makeup of aluminum chlorate to the industrial uses of barium sulfate, this narrative unravels the complexities of these compounds, shedding light on their significance in various fields.
Aluminum Chlorate and Barium Sulfate

Aluminum chlorate, with the chemical formula Al(ClO3)3, is a white crystalline solid. It has a molecular structure consisting of an aluminum ion (Al3+) surrounded by three chlorate ions (ClO3-). Aluminum chlorate is highly soluble in water and has a pungent odor.
Barium sulfate, on the other hand, has the chemical formula BaSO4. It is a white, odorless, and tasteless powder. The molecular structure of barium sulfate consists of a barium ion (Ba2+) surrounded by four sulfate ions (SO42-). Barium sulfate is insoluble in water and has a high density.
Aluminum Sulfate and Barium Chlorate
Aluminum sulfate, with the chemical formula Al2(SO4)3, is a white crystalline solid. It has a molecular structure consisting of two aluminum ions (Al3+) surrounded by three sulfate ions (SO42-). Aluminum sulfate is highly soluble in water and has a sour taste.
Barium chlorate, on the other hand, has the chemical formula Ba(ClO3)2. It is a white crystalline solid. The molecular structure of barium chlorate consists of a barium ion (Ba2+) surrounded by two chlorate ions (ClO3-). Barium chlorate is soluble in water and has a bitter taste.
Chemical Reactions: Aluminum Chlorate + Barium Sulfate Aluminum Sulfate + Barium Chlorate
| Reactants | Products | Reaction Conditions |
|---|---|---|
| Aluminum chlorate + Barium sulfate | Aluminum sulfate + Barium chlorate | Aqueous solution |
| Aluminum sulfate + Barium chlorate | No reaction | Aqueous solution |
Applications
Aluminum Chlorate, Aluminum chlorate + barium sulfate aluminum sulfate + barium chlorate
- Textile industry as a mordant
- Water treatment as a coagulant
- Paper industry as a bleaching agent
Barium Sulfate
- Medical imaging as a contrast agent
- Paint industry as a filler
- Rubber industry as a reinforcing agent
Safety Considerations
Aluminum Chlorate, Aluminum chlorate + barium sulfate aluminum sulfate + barium chlorate
Aluminum chlorate is a strong oxidizing agent and can be explosive if not handled properly. It should be stored in a cool, dry place away from flammable materials. Contact with skin and eyes should be avoided.
Barium Sulfate
Barium sulfate is generally considered to be non-toxic, but it can cause respiratory irritation if inhaled. It should be handled with care and dust masks should be worn when working with it.
FAQ Explained
What are the key differences between aluminum chlorate and barium sulfate?
Aluminum chlorate is a water-soluble salt, while barium sulfate is insoluble in water. Aluminum chlorate is a strong oxidizing agent, while barium sulfate is chemically inert.
What are the main industrial applications of aluminum sulfate?
Aluminum sulfate is primarily used in water treatment, papermaking, and textile manufacturing.